CLINICAL TRIALS AND RESULTS
JOHNS HOPKINS UNIVERSITY INVESTIGATOR SPONSORED CLINICAL PROOF OF CONCEPT TRIAL
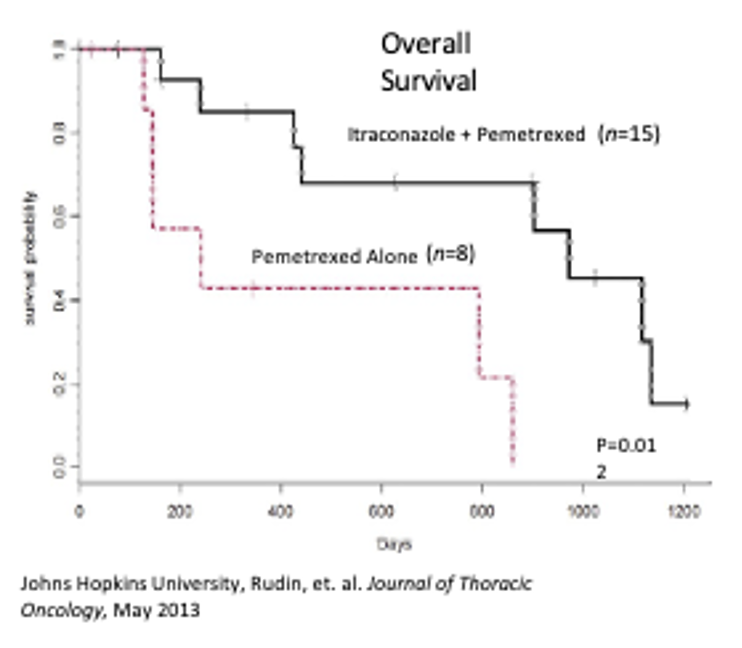
A randomized controlled phase 2 study of generic oral itraconazole in patients with metastatic non-squamous non-small cell lung cancer (NS NSCLS). A two-arm study: combination with standard dose of second-line Pemetrexed.
Conclusions consisted of an improved median survival from 8 months to 32 months, it was well tolerated with grade 3 toxicity related to Pemetrexed (in 5 patients total). Itraconazole as an oral therapy may extend survival in patients with an unmet need.