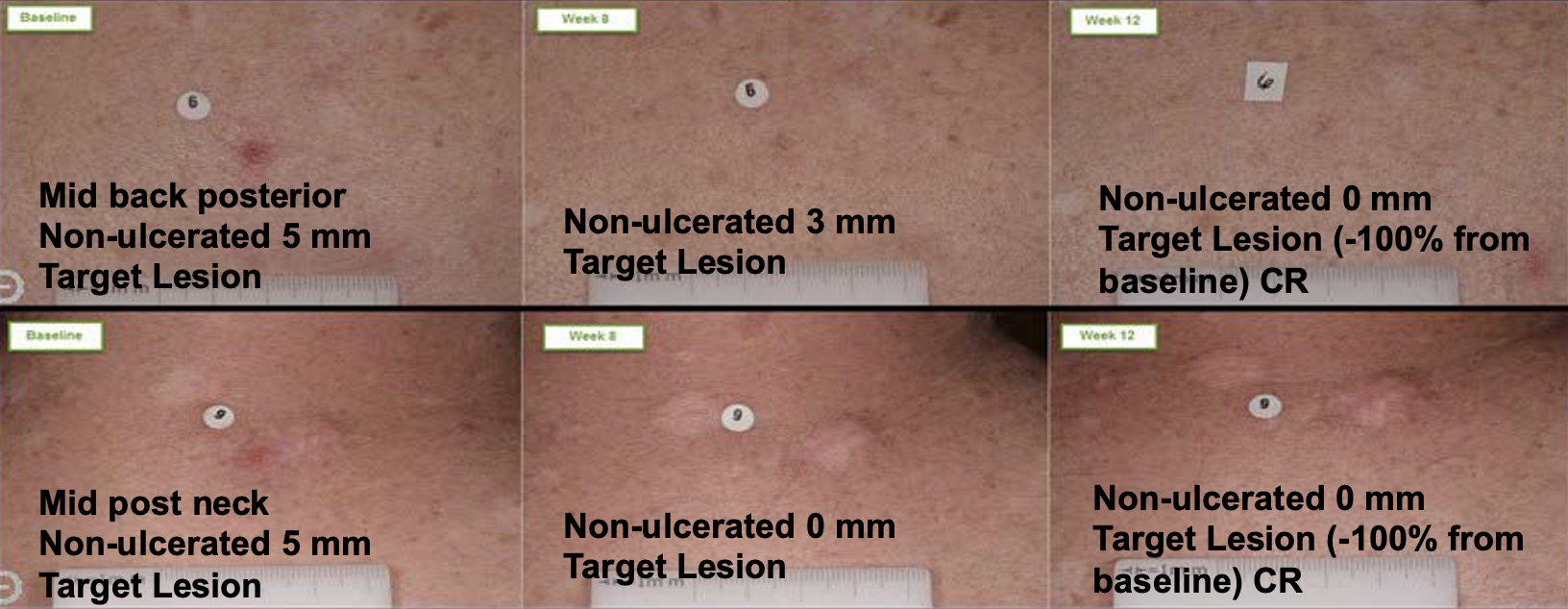
FORWARD LOOKING
Now that IND has been transferred to the dermatology division of the FDA, we can pursue regulatory guidance for marketing approval for BCCNS and identify what clinical trial data is needed to support sNDA for high-frequency basal cell carcinoma indication.
Our top priority currently is negotiating with Johns Hopkins University in hopes of gaining exclusive rights to their related patents ‘Hedgehog Pathway Antagonists to Treat Disease’ & ‘Angiogenesis Inhibitors’.
We are also working behind the scenes with CDMO to create alternative patentable formulation that deliver the desired PK profile to ensure we are bringing our best possible product to market.