Scientific Advisory Board
At the forefront of the Inhibitor Therapeutics, Inc. (OTCQB:INTI) pipeline is the use of Itraconazole to treat basal cell carcinomas in Basal Cell Carcinoma Nevus Syndrome (BCCNS) aka Gorlin Syndrome. This rare hereditary cancer syndrome is characterized by patients with hundreds to thousands of basal cell carcinomas. BCCNS is an orphan disease with approximately 11,000 patients in the USA. It is an autosomal dominant disorder that may affect every organ system.
Currently the first line treatment for BCCs on exposed areas such as the face and neck is Mohs Surgery which becomes increasingly challenging and leads to permanent scarring and disfiguration. As patients age as they may undergo hundreds of surgeries. Inhibitor believes that itraconazole has clinically meaningful effects on the BCCs, which led us to recruit a team of five experienced Mohs surgeons to form a Scientific Advisory Board (SAB) to critically review the outcomes of Inhibitor’s phase 2b trial in BCCNS. They are academically affiliated, well-respected leaders in the practice of treating basal cell carcinomas and other skin cancers The purpose of the SAB is to assess the clinical benefits of adding itraconazole to the therapeutic options for the basal cell carcinomas in these patients.
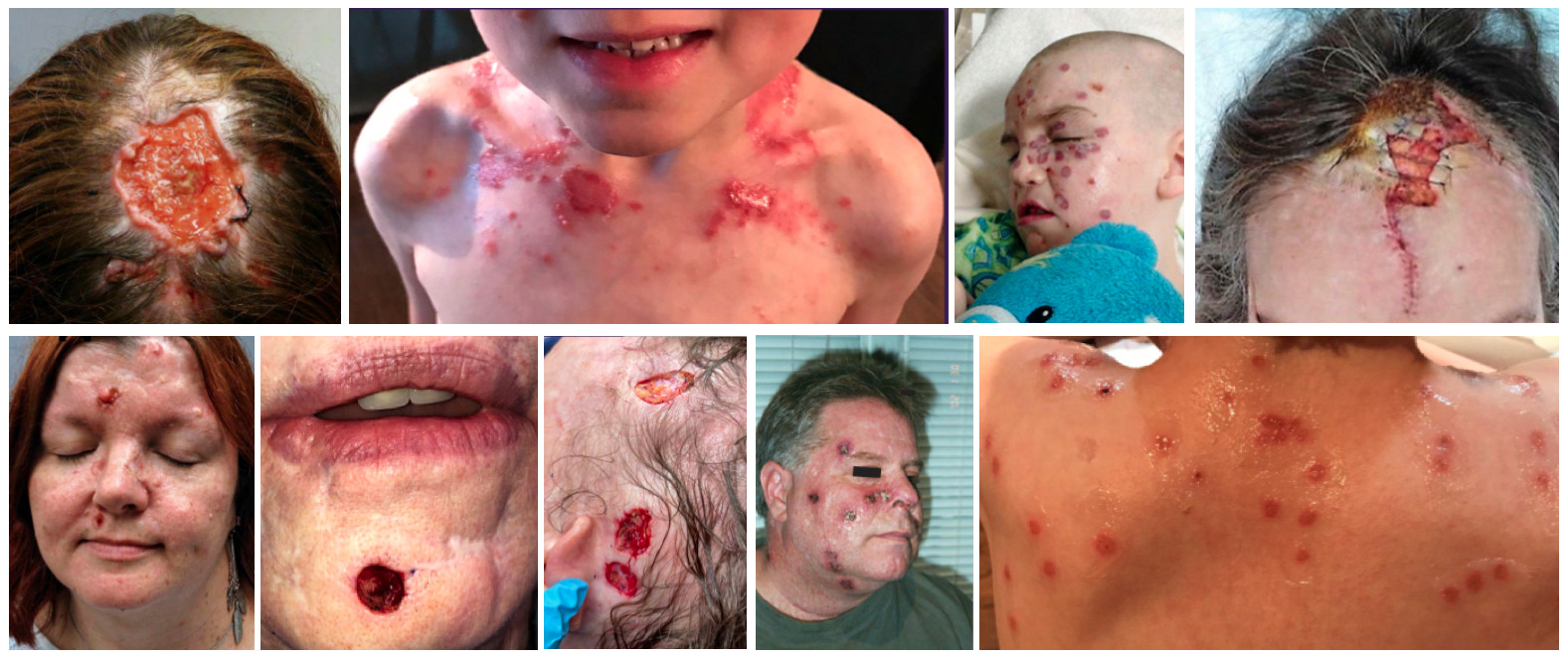
Patients suffering from BCCNS (via Gorlin Syndrome Alliance, Voice of Patient Gallery)
The initial focus of the SAB is a review of the final clinical outcomes of INTI’s phase 2b study of itraconazole in BCCNS. Due to a recently resolved dispute with the former supply partner, the trial results are now available but have not been publicly released, nor shared with the trial investigators, or filed with the FDA for guidance.
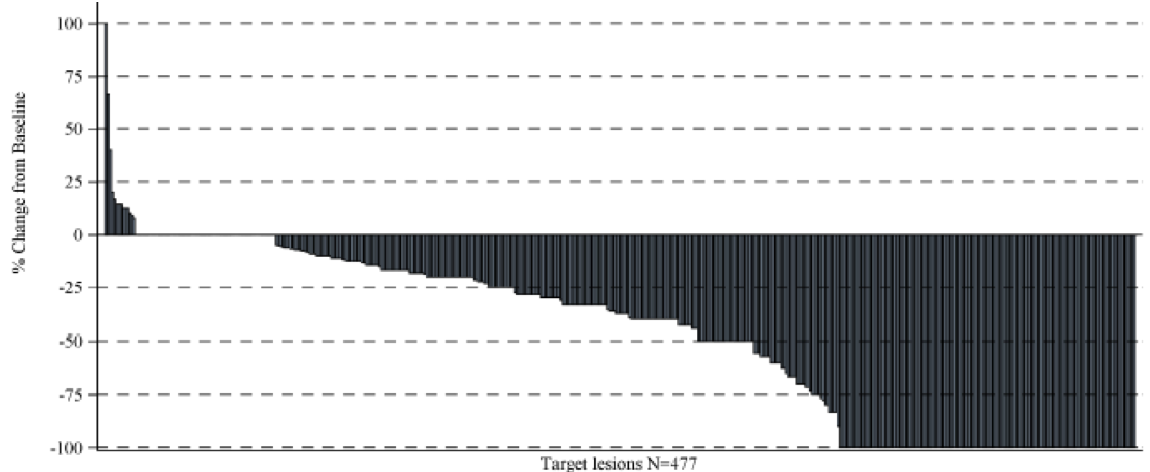
Our Phase 2b SCORING Trial completed during 2018 assessed 477 pre-existing surgically eligible target lesions across 38 patients. Unlike virtually all other disseminated cancers that have a single primary with metastatic lesions, the standard measure of response is RECIST (Response Criteria in Solid Tumors) which are size criteria based upon an analysis of response by patient. RECIST criteria were developed for primary tumors with or without metastatic lesions. Unlike other cancers, in BCCNS, every patient had multiple tumors (on average over 12) and all the tumors are primaries. In this cancer syndrome, the 477 surgically eligible tumors each represented a unique basal cell carcinoma not a metastatic lesion. The median duration of treatment was 213 days (ranging from 38 to 1117 days)., It was observed that 275 of the 477 surgically eligible lesions (57.6%) met a clinically meaningful degree of reduction of 30% or more with 130 lesions (27.3%) resolving completely and 145 tumors (29.7%) shrinking in size by at least 30% and 64 lesions (13.4%) were stabilized. A total of 13 new surgically eligible BCCs occurred in 8 patients between Week 16 and Week 109, wherein 6 patients developed a new single lesion and 2 subjects developed multiple new lesions. The median duration of response was 540 days.
The adjacent graph shows the ‘Change from Baseline for All Target Lesions’ finding that across the 477 lesions reductions of any size from baseline was reported in 399 lesions, 64 had no change, and 14 increased in size.
The itraconazole oral treatment demonstrated a level of safety commensurate with the known profile and was generally well tolerated. Only one patient discontinued the drug because of progressive disease. There were no treatment-related serious adverse events.
Patients with BCCNS are predominantly concerned with the high number of surgical interventions required during their lifetime and the disfigurement that results from these procedures, particularly on areas of the body such as the face that are exposed to the public. Therefore, a therapy without significant toxicity that can reduce the number of lesions that require a scar producing intervention will fulfill an unmet need for these patients. The review of the Phase 2b Clinical Study Report by our Scientific Advisory Board will focus on whether the SCORING trial is sufficiently clinically meaningful to warrant filing itraconazole for a New Drug Application (“NDA”) as the first and only drug to be approved to treat BCCNS.
The Gorlin Syndrome Alliance (a non-for-profit independent patient advocacy organization) (GSA) conducted an EL-PFDD (externally led patient focused drug development) meeting on October 8, 2021. This was conducted to help advance the GSA’s mission to thoughtfully support, comprehensively educate, and aggressively seek the best treatments and a cure for those affected by Gorlin Syndrome. A majority of participants with Gorlin Syndrome at the meeting clearly felt that short of a cure, reducing the tumor burden of BCCs by at least 30% would be a meaningful clinical endpoint.
Readers are encouraged to visit the Gorlin Syndrome Alliance website (www.gorlinsyndrome.org) with special attention to the Externally led, Patient Focused Drug Development white paper (https://gorlinsyndrome.org/voice-of-patient-report/). This report reflects the challenges and needs of patients with this syndrome.
SAB MEMBERS

Dr. Elizabeth M. Billingsley, MD.
Head of the SAB, Dr. Billingsley is a Professor of Dermatology with Penn State Health Hershey Medical Center, and Penn State College of Medicine. She received her undergraduate degree from Cornell University and her medical degree from Penn State University College of Medicine. She is a Mohs micrographic surgeon with more than 30 years’ experience in Mohs Surgery and skin cancer management. She also has performed numerous clinical trials related to skin cancer. Dr Billingsley is a past president of the American College of Mohs Surgery. She is affiliated with the Gorlin Syndrome Alliance and is a member of their Medical and Scientific Advisory Committee.

Dr. Marc D. Brown, MD
Dr. Brown developed an interest in dermatology after an extensive education. He completed a dermatology residency at the University of Michigan in 1987. Following this came a two-year fellowship training for Mohs Surgery and Cutaneous Oncology. He joined the faculty at the University of Rochester Medical Center in 1989 and is a tenured professor of Dermatology and Oncology and is a member of the Wilmot Cancer Center. He served as the director of the Dermatology Residency Program and the Mohs Surgery Fellowship Program at the Rochester Medical Center. He has been included in the Best Doctors in America directory and has published a lengthy list of literature. Dr. Brown performs Mohs surgery on over 2,000 patients per year and has performed a total of more than 50,000 Mohs procedures.

Dr. Allison Vidimos, RPh, MD.
Dr. Vidimos was appointed Chairman of the Department of Dermatology at Cleveland Clinic 2005 and Vice Chairman of the Dermatology and Plastic Surgery Institute in 2006. She was appointed Professor of Dermatology, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University in 2011. She became the program director of the Micrographic Surgery and Dermatologic Oncology fellowship in 2013. She was a member of the Scientific Assembly Committee and Membership Committee for the American Academy of Dermatology (AAD) 2012-17. She was elected to the Board of Directors of the AAD in 2022. She served as President of the American College of Mohs Surgery (ACMS) in 2017-2018. Dr. Vidimos received the Frederic Mohs Lifetime Achievement Award in 2021. She was appointed to the Board of Directors for the American Board of Dermatology for 2019-2027 and is a member and Chairman of the board question writing committee for dermatologic surgery. She was elected to the Board of Directors of the Ohio Dermatological Association (ODA) in 2019-21 and is President of ODA 2022-23. Her clinical practice and research encompass skin cancer prevention, diagnosis and treatment, and patient safety.

Dr. Sean R. Christensen, MD, PhD.
Dr. Christensen is an Associate Professor of Dermatology; Director of Resident Education in Dermatologic Surgery; Director of Dermatologic Surgery at Yale Dermatology-Branford. Dr. Christensen has been practicing dermatologic surgery since completing training in 2013. His surgical specialization includes Mohs surgery, treatment of early-stage melanoma, and surgical reconstruction. Additionally, Dr. Christensen focuses on complex skin cancer issues such as field characterization, preventative strategies in high-risk patients and management of advanced or aggressive skin cancer. Dr. Christensen has published extensively on skin cancer pathogenesis and treatment and has experience in clinical trials for basal cell carcinoma. He is a frequent lecturer at national meetings for organizations such as the American Academy of Dermatology and the American College of Mohs surgery, and currently serves as the Treasurer for the International Transplant Skin Cancer Collaborative.

Dr. Ian Maher, MD
Dr. Maher is a Professor of Dermatology and Director of Dermatologic Surgery at University of Minnesota. He is board certified in Mohs surgery, specializing in the treatment of a broad range of common and rare skin cancers as well as post-skin cancer reconstruction. Dr. Maher has served on the Boards of multiple national Dermatologic organizations. He has published over 100 peer-reviewed articles.



